| 2000 |
Oct 2000
Nov 2000 |
- Biometrix Technology Inc. established
- Approval of Korean Venture Company by SMBA |
|
| 2001 |
Feb 2001 |
- Moving into Chuncheon Bioventure Innovation Center |
|
| 2002 |
Jul 2002 |
- BMT R&D center established
|
 |
| 2003 |
May 2003 |
- Approval of Korean Venture Company by KIBO |
 |
| 2004 |
Dec 2004 |
- Awarded Ministry of commerce, Industry and Energy on 100th
- outstanding issues(Biochip manufacturing technology |
 |
| 2005 |
May 2005 |
- Approval of Korean Venture Company by KIBO |
 |
| 2007 |
May 2007
Jun 2007
Jun 2007
Jul 2007
Nov 2007
Dec 2007 |
- Exhibition Show at BIO2007(USA, Boston)
- Approval of INNO-BIZ company by SMBA
- Approval of Korean Venture Company by KIBO
- Exhibition Show at American Association of Clinical Chemistry (AACC) 2007
- Exhibition Show at Compamed 2007(Germany, Dusseldorf)
- Awarded a prize of Korean FBA Best Bioventure company |
 |
| 2008 |
Jul 2008
Aug 2008
Nov 2008 |
- Exhibition Show at American Association of Clinical Chemistry (AACC) 2008
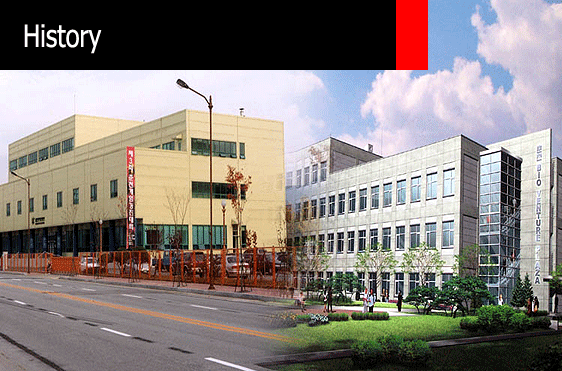
- Moving into Bio Venture Plaza
- Exhibition Show at Compamed 2008(Germany, Dusseldorf) |
 |
| 2009 |
Feb 2009
Jul 2009
Oct 2009
Nov 2009
Nov 2009 |
- Approval of Korean Venture Company by KIBO
- Exhibition Show at American Association of Clinical Chemistry (AACC) 2005
- Approved an ISO 13485:2003 by TUV SUD(DNA kit & analyzer)
- CE Certification for BMT HPV 9G DNA Kit
- Exhibition Show at MEDICA 2009(Germany, Dusseldorf) |
 |
| 2010 |
Jan 2010
Feb 2010
Apr 2010
Apr 2010 |
- Approval of specification and test methods for BMT HPV 9G DNA Kit (KFDA)
- Approval of Korean Venture Company by KIBO
- CE Certification for BMT HPV 9G MEMBRANEN DNA Kit
- CE Certification for 1-D Scanner |